What is ONC201?
ONC201 is an oral, small-molecule drug that selectively targets and destroys aggressive cancer cells while leaving healthy cells unharmed. Currently in clinical trials, it has shown remarkable promise for recurrent glioblastoma (GBM), diffuse intrinsic pontine glioma (DIPG), and other H3 K27M-mutant cancers, diseases with few effective treatments and devastating survival rates.
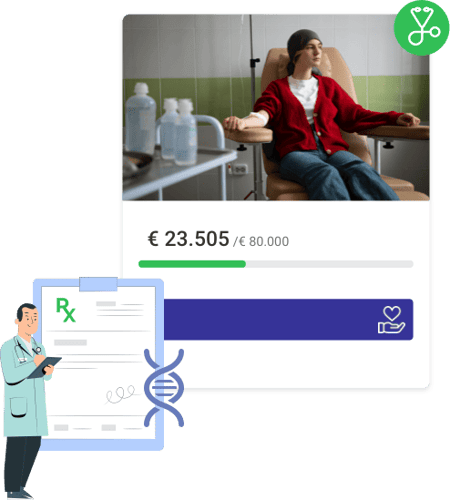
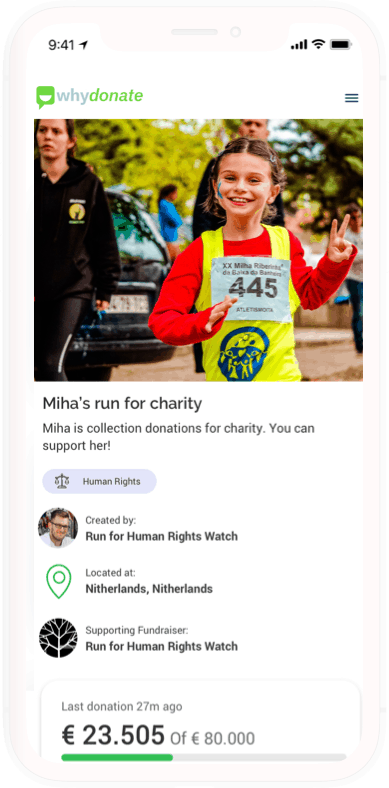
Cheto’s fundraiser raised over €36,000, proof that hope, community, and WhyDonate can change lives. Start your own fundraiser today and let others rally behind your fight.
Who is Eligible for ONC201 Treatment?
Clinical trials are crucial to determine the effectiveness of a clinical strategy, drug, treatment, or tool. Eligibility for ONC201 clinical trials typically includes adults (18 years or older) with specific types of glioma who have completed standard frontline radiotherapy within a defined period (usually 2–6 weeks before randomization).
Patients should not have primary spinal tumors and must not be pregnant or breastfeeding during treatment or for a set period after the last dose.